|
Film
deposition of BSA
Bovine
Serum Albumin is the main component of blood proteins for all animals and
plays a great role in the body. BSA is a midsized protein with a molecular
weight of 66 KDa.
BSA has well defined functions and features:control of
osmotic blood pressure; transport and storage of nutrients; good binding
abilities for water and cations (K+, Na+,Ca++);
fatty acid complexation and transport; drugs complexation; adhesion at both
hydrophilic and hydrophobic surfaces. BSA is usually employed as a basic
monolayer binding to different acids to form soluble and/or insoluble
complexes depending on the acid types and their pH, in order to realize
human body implants. Bovine Serum Albumin thin
films were deposited by Matrix-Assisted Pulsed
Laser Evaporation. The primary and secondary structure of the protein were
analysed, since the functionality of the protein is determined by both
structures.
The deposition was perfomed after dilution of the protein
into two different solvents (deionized water and PBS). Both the solvents
gave good results since from biological tests (SDS-PAGE) and structural
characterizations (FTIR) the integrity of the whole protein and the
characteristic vibrational bands in the infrared region, respectively, were
preserved. In fact, by comparison FTIR absorbance spectra of the BSA bulk
and of the MAPLE deposited films, all the characteristic peaks were revealed
confirming the integrity of the protein secondary structure.
The spectra show that the α-helix structure is dominant,
as indicated by the absence of the peak related to the β-sheet protein
configuration (located at 1628 cm-1). The primary structure of
BSA was ensured from SDS-PAGE test.
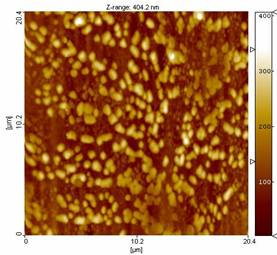 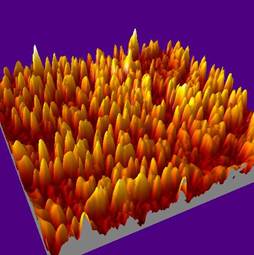
Fig. 1: (a) AFM and (b) 3-D extrapolation images of a
BSA thin film
The Figure 1 a) shows the AFM images of a typical BSA
film deposited with a fluence of 150 mJ/cm2 and a BSA
concentration of 1% wt in PBS, while in Fig. 1 b) 3-D reconstruction is
reported. The film is uniformly covered by spheroid-like structures
confirming the well known tendency of BSA protein to aggregate into
macromolecular assemblies.
|
